| Air |
| WATER |
|
| SOLID WASTE |
| AGRICULTURE |
|
| CLIMATE |
| DEMOGRAPHY |
| BIODIVERSITY |
|
|
|
 |
| Save Ganga Save Dolphin |
|
Pure air
consists of roughly 78.09% nitrogen, 20.94% Oxygen, 0.93% Argon, 0.0318% carbon dioxide and small amounts of other gases (by volume). Air also contains a variable amount of water vapor, on average around 1%.

But with growth of urbanization and industrialization a lot of other elements have been added to pure air. This resulted in the increase of pollution. In order to prevent control and abate air pollution, the Air (Prevention and Control of Pollution) Act was enacted in 1981. According to Section 2(b) of Air (Prevention and control of pollution) Act, 1981 'air pollution' has been defined as 'the presence in the atmosphere of any air pollutant'. As per Section 2(a) of Air (Prevention and control of pollution) Act, 1981' air pollutant' has been defined as 'any solid, liquid or gaseous substance [(including noise)] present in the atmosphere in such concentration as may be or tend to be injurious to human beings or other living creatures or plants or property or environment'. Therefore ambient air quality standard is developed as a policy guideline that regulates the effect of human activity upon the environment so that pollutant emission into the air can be regulated.
National Ambient Air Quality Standards (NAAQS)
| The objectives of air quality standards are; |
 |
To indicate the levels of air quality necessary with an adequate margin of safety to protect the public health, vegetation and property; |
 |
To assist in establishing priorities for abatement and control of pollutant level; |
 |
To provide uniform yardstick for assessing air quality at national level; |
 |
To indicate the need and extent of monitoring programme. |
The revised National Ambient Air Quality Standards notified on November 2009 is depicted below
Revised National Ambient Air Quality Standards (NAAQS)
[NAAQS Notification dated 18th November, 2009]
| S. No |
Pollutants |
Time Weighted Average |
Concentration in Ambient Air |
Methods of Measurement |
| Industrial, Residential, Rural and other Areas |
Ecologically Sensitive Area (notified by Central Government) |
| 1. |
Sulphur Dioxide (SO2), µg/m3 |
Annual * |
50 |
20 |
1. Improved West and Gaeke
2. Ultraviolet Fluorescence |
| 24 Hour ** |
80 |
80 |
| 2. |
Nitrogen Dioxide (NO2), µg/m3 |
Annual * |
40 |
30 |
1.Modified Jacob & Hochheiser
(Na-Arsenite)
2.Chemiluminescence |
| 24 Hours** |
80 |
80 |
| 3. |
Particuate Matter (Size<10 µm) or PM10 µg/m3 |
Annual * |
60 |
60 |
1.Gravimetric
2. TOEM
3. Beta attenuation |
| 24 Hours** |
100 |
100 |
| 4. |
Particulate Matter (Size<2.5 µm) or PM2.5 µg/m3 |
Annual * |
40 |
40 |
1.Gravimetric
2. TOEM
3. Beta attenuation |
| 24 Hours** |
60 |
60 |
| 5. |
Ozone (O3), µg/m3 |
8 hours ** |
100 |
100 |
1.UV photometric
2.Chemiluminescence
3.Chemical Method |
| 1 hours** |
180 |
180 |
| 6. |
Lead (Pb), µg/m3 |
Annual * |
0.50 |
0.50 |
1.AAS/ICP Method after sampling
using EPM 2000 or equivalent filter
paper
2.ED-XRF using Teflon filter |
| 24 Hour** |
1.0 |
1.0 |
| 7. |
Carbon Monoxide (CO), mg/m3 |
8 Hours ** |
02 |
02 |
Non dispersive Infra Red (NDIR)
Sepectroscopy |
| 1 Hour** |
04 |
04 |
| 8. |
Ammonia (NH3), µg/m3 |
Annual * |
100 |
100 |
1. Chemiluminescence
2.Indophernol blue method |
| 24 Hour** |
400 |
400 |
| 9. |
Benzene (C6H6), µg/m3 |
Annual * |
05 |
05 |
1.Gas chromatography based
continuous analyzer
2.Adsorption and Desorption
followed by GC analysis |
| 10. |
Benzo(a)Pyrene (BaP)-particulate phase only, ng/m3 |
Annual * |
01 |
01 |
Solvent extraction followed by HPLC/GC
analysis |
| 11. |
Arsenic (As), ng/m3 |
Annual * |
06 |
06 |
AAS/ICP Method after sampling using
EPM 2000 or equivalent filter paper |
| 12. |
Nickel (Ni), ng/m3 |
Annual * |
20 |
20 |
AAS/ICP Method after sampling using
EPM 2000 or equivalent filter paper |
* Annual Arithmetic mean of minimum 104 measurements in a year at a particular site taken twice a week 24 hourly at uniform interval.
** 24 Hourly 08 hourly of 01 monitored values, as applicable shall be complied with 98% of the time in a year. 2% of the time, they may exceed the limits but not on two consecutive days of monitoring.
National Air Quality Monitoring Programme(NAMP)
Central Pollution Control Board initiated National Ambient Air Quality Monitoring(NAAQM) programme in the year 1984 with only seven monitoring stations in the country. Further, it has been strengthen by increasing the number of monitoring stations with two monitoring stations in currently existing Bihar. The one monitoring station is situated at Beltron Bhawan, Shastri Nagar, Bailey Road, Patna and the another at Gandhi Maidan, Patna and the monitoring work is being carried out by the State Pollution Control Board. Later, the programme NAAQM was renamed as National Air Quality Monitoring Programme(NAMP) and their objectives are:
 |
To determine status and trends of ambient air quality |
 |
To ascertain whether the prescribed ambient air quality standards are violated. |
 |
To identify Non-attainment Cities; To obtain the knowledge and understanding necessary for developing, preventive and corrective measures; and |
 |
To understand the natural cleansing process undergoing in the environment through pollution dilution, dispersion, wind based movement, dry deposition, precipitation and chemical transformation of pollutants generated. |
The following parameters are being monitored under the NAMP in Bihar
 |
Sulphur Dioxide(SO2). |
 |
Oxides of Nitrogen(NOx). |
 |
Respirable Particulate Matter(RSPM) or ,PM10. and |
 |
Suspended Particulate Matter(SPM).
|
The Central Pollution Control Board has classified the air quality into the following four broad categories
Pollution Level Classification
Pollution level
|
Annual Mean Concentration Range (µg/m3) |
Industrial, Residential, Rural & other areas |
Ecologically Sensitive Area |
SO2 |
NO2 |
PM10 |
SO2 |
NO2 |
PM,10 |
Low (L) |
0-25 |
0-20 |
0-30 |
0-10 |
0-15 |
0-30 |
Moderate (M) |
26-50 |
21-40 |
31-60 |
11-20 |
16-30 |
31-60 |
High (H) |
51-75 |
41-60 |
61-90 |
21-30 |
31-45 |
61-90 |
Critical (C) |
>75 |
>60 |
>90 |
>30 |
>45 |
>90 |
Air Quality Trend of Patna We live in
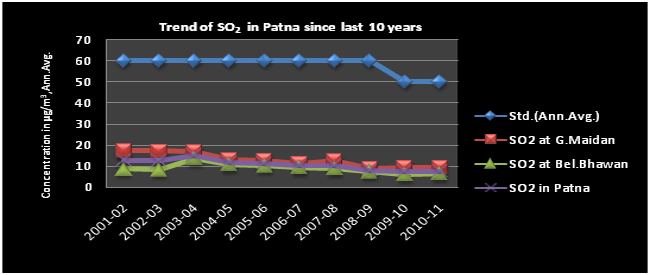
Trend of SO2
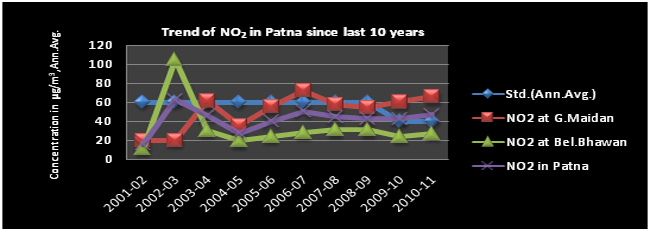
Trend of NO2
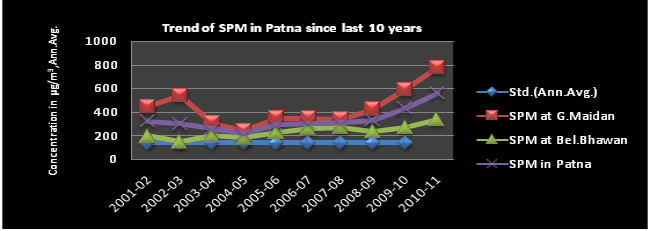
Trend of SPM
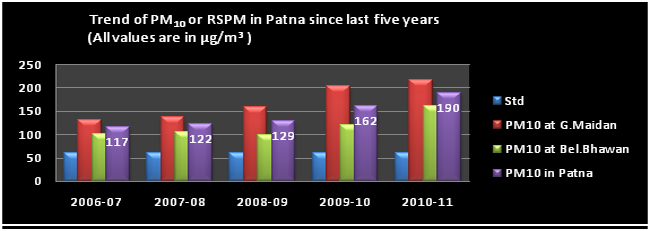
Trend of PM10
Analysis of last ten years air quality data shows a more or less stable trend for SO2 & NO2 however, SPM and RSPM show an increasing trend and exceeds the NAAQS.
Background of Parameters
Sulphur dioxide (SO2) is a colorless, soluble gas with a characteristic pungent smell. It is a chemical compound produced by volcanoes and in various industrial processes and are also a precursor to particulates in the atmosphere. Its natural source is volcanic eruption (67%) and anthropogenic sources are combustion of fossil fuel (Coal, heavy fuel oil in thermal power plants and factories), paper Industry, Extraction & distribution of fossil fuels, smelting of metals (sulfide ores to produce copper, lead and zinc), petroleum refining, combustion process in diesel, petrol, natural gas driven vehicles. SO2 is always accompanied by a little amount of SO3 and in ambient air it can also affect human health. It also causes visibility impairment. It is considered more harmful when particulate and other pollution concentrations are high. The atmospheric SOX are converted into H2SO4 by the processes of photochemical reactions and ultimately come to the earth surface in the form of acid rain.
Oxides of Nitrogen(NOX) are a generic term for a group of highly reactive gases that contain nitrogen and oxygen in varying amounts(nitric oxide-NO and nitrogen dioxide-NO2). NO is a colourless, odourless gas, but Nitrogen dioxide (NO2) is a reddish-brown toxic gas with a characteristic sharp, biting odor and is a prominent air pollutant. Oxides of nitrogen are formed during combustion processes at high temperatures from the oxidation of nitrogen in air. NOX are emitted as nitrogen oxide (NO) which is rapidly oxidized to more toxic nitrogen dioxide (NO2). Sources of nitrogen oxides includes lightning, forest fires, bacterial activity of soil as natural source and vehicles, industrial processes that burn, high temperature combustion (internal combustion engines, fossil fuel-fired power stations, industrial, burning of bio-mass) and fossil fuels are anthropogenic sources. NO2 irritates the nose and throat increase susceptibility to respiratory infections. In addition, NOX is a potent and selective vasodilator in pulmonary arterial hypertension. Oxides of nitrogen react with Volatile Organic Compounds (VOCS) to form ground level ozone. They also react to from nitrates, acid aerosols. Almost all NOX is emitted as NO, which is rapidly oxidized to more toxic NO2. They also contribute to nutrient overload that deteriorates water quality. The atmospheric NOX also converted into HNO3 by the processes of photochemical reactions and ultimately precipitated as nitrate salts in either rainfall(as acid rain) or as dust and then come to the earth surface.
Particulate Matter: Small, solid particles and liquid droplets are collectively termed as particulates. These are present in the atmosphere in large numbers. Particle pollution, also called particulate matter or PM, is a complex mixture of extremely small particles and liquid droplets in the air. Their most important property is size, which range in the atmosphere from a diameter of 0.0002μ to a diameter of 500μ.It is known that particulate matter burden of air is less important than the chemical nature and size of the particles. There are various particulates which may present in the atmosphere, such as Fine Particles(less than 100 μ in diameter),Coarse Particles(greater than 100 μ in diameter),Dust(solid particles larger than colloidal-size particles in atmosphere, which are known as Aerosols, contains particles of size ranging from 1 to 200 μ),Aerosol(air borne suspensions either solid or liquid and size generally ⩽1μ),Smoke(may be either liquid or solid, particle size ranging from 0.01 to 1 μ),Fumes(solid particles released from chemical or metallurgical processes, size ranging from 0.1 to 1 μ), Ultrafine particles (UFP)[size ⩽ 0.1µm] and Mist ,Fog & Smog(size generally less than 10 μ).
Particle pollution also is the main cause of environmental problems. When breathed in, these particles can reach the deepest regions of the lungs. Exposure to particle pollution is linked to a variety of significant human health problems.
Suspended Particulate Matter (SPM) are the bigger than coarse particles, these settle down fast and does not reach the respiratory tract. Therefore they have less adverse effect on health. As a result it has not been included in the revised standard. The factors responsible for high SPM are extensive urbanization and construction activities, vehicular, vehicular population increase, frequent use of captive power generation unit/domestic generation, extensive use of fossil fuel and biomass (wood, leaves etc.) as well as particulate contribution from biological debris. The SPM is also affected with the seasonal variation.
The Central Govt. first issued standards for suspended particulate matter in 1994. In November 2009, the Central Govt. revised the 1994 standards in which SPM was revoked and fine particles (PM2.5, size 2.5 micrometers in diameter and smaller) has been included.
Respirable Suspended Particulate Matter,[RSPM or Particuate Matter (Size <10 µm) or PM10] consist of particles with a diameter up to 10µm. The major constituents of RSPM are organic and elemental carbon, metals/elements + like silicon. magnesium, iron, ion ions like sulphates, nitrates, ammonium etc. PM10 can be formed by physical processes of crushing. grinding and abrasion of surfaces. Mining and agricultural activities are some of the sources of large size particles. The anthropogenic sources are coarse particles and are produced by the mechanical break-up of large solid particles, windblown dust such as road dust, fly ash, soot, agricultural processes, physical processes of crushing, grinding and abrasion of surfaces, photo chemically produced particles, such as those found in urban haze, pollen grains, mould spores, and plant and insect parts and anthropogenic sources are combustion of fossil fuel (Coal, heavy fuel oil in thermal power plants, office, factories), paper Industry, extraction & distribution of fossil fuels, smelting of metals (sulfide ores to produce copper, lead and zinc), Petroleum refining. Combustion process in diesel, petrol, natural gas driven vehicles PM10 can settle in the bronchi and lungs and cause health problems like respiratory illness, visibility impairment, aggravate existing heart and lung diseases. It also causes visibility reduction.
Particulate Matter (Size <2.5 µm) or PM2.5.
These particles are respirable fraction composed mainly of carbonaceous materials (organic and elemental). inorganic compounds (sulfate, nitrate, and ammonium), and trace metal compounds (iron, aluminium, nickel, copper, zinc, and lead) penetrates deeper into the lungs increases respiratory symptoms, causes irritation of the airways, coughing, or difficulty breathing, decreases lung function; aggravates asthma, chronic bronchitis, irregular heartbeat, nonfatal heart attacks, premature death in people death in people with heart of lung disease
Ammonia(NH3)
Molecular formula-NH3; Nature: non-poisonous and basic in nature. It occurs in free state and is present in atmospheric air, natural water (traces),sewage water(high proportion),decaying plant and animal tissues, urine and excreta of animal. It is a colorless gas, lighter than air, that fumes strongly in air and highly soluble in water. It has a sharp burning smell, which brings tears to the eyes and it can damage respiratory system. It increases the heart beat in large doses. Ammonia is produced naturally in large quantities, but it is oxidized to NO2 and finally to HNO3 which is washed out by rains or as precipitate of sulphate or nitrate.
Carbon Monoxide(CO)
Molecular formula-CO. It is a colorless, odorless, tasteless gas, as heavy as air and not soluble in water. It is formed by incomplete combustion of fossil fuel like coal and petroleum, or other organic matter. Automobiles are the commonest source of carbon monoxide pollution in the cities. Other common sources are oil refineries, metallurgical operations and other internal combustion engines. It is a major pollutant for man and animals as it combines with blood hemoglobin much faster than oxygen. Because CO gradually gets oxidized to CO2, therefore it is not a long range global threat, but in busy area its content rises very much and result reduction in hemoglobin, feeling of headeque and suffocation. Vegetation, Ocean and Soil are the major natural sink of carbon monoxide.
Benzene(C6H6)
The chemical formula for benzene is C6H6, and it has a molecular weight of 78.11 g/mol. Benzene occurs as a volatile,colorless, highly flammable liquid that dissolves easily in water. Benzene has a sweet odor. The vapor is heavier than air and may travel along the ground; distant ignition possible. Reacts violently with oxidants, nitric acid, sulfuric acid and halogens causing fire and explosion hazard. Attacks plastic and rubber. Its vapor/air mixtures are explosive and there is risk of fire and explosion. Benzene is used as a constituent in motor fuels; as a solvent for fats, waxes, resins, oils, inks, paints, plastics, and rubber; in the extraction of oils from seeds and nuts; and in photogravure printing. It is also used as a chemical intermediate. Benzene is also used in the manufacture of detergents, explosives, pharmaceuticals, and dyestuffs.
Benzene is a toxic air pollutant that can cause serious health effects including cancer. Benzene is found in the ambient air due to emissions from burning of coal and oil, evaporation from gasoline service stations, and motor vehicle exhaust. Other sources include wood smoke and cigarette smoke. Tobacco smoke also contains benzene and accounts for individual exposure to benzene. These sources contribute to elevate level of benzene in the ambient air, which may subsequently be breathed by the public. Benzene is very toxic to aquatic organisms also.
Benzo(a)Pyrene (BaP)-particulate phase only, ng/m
Benzopyrene or Benzo(a)Pyrene (BaP) is an organic compound with the molecular formula C20H12,high molecular weight, density- 1.24 g/cm3 (25 °C), and 5-ring PAH. BaP is one of the polycyclic aromatic hydrocarbons (PAHs) formed when gasoline(automobile exhaust fumes, especially from diesel engines), garbage or any animal or plant materials burn incompletely or incomplete combustion of organic materials, such as wood or fossil fuels and from coke oven emissions. BaP and PAHs are ubiquitous environmental contaminants and highly carcinogenic. People may be exposed to BaP from air, water, soil, cigarette and other plant product smoke, food and some work environments through inhalation, ingestion and skin contact. Exposure to PAHs may cause harmful health effects.
Ozone(O3)
The upper layer of atmosphere, stratosphere consists of considerable amount of ozone (O3),which protects us from the harmful ultraviolet radiation coming from the sun. Therefore, it is important to maintain the ozone shield. Despite having three atoms of oxygen in ozone, it is harmful in lower layers of the atmosphere near earth’s surface. Ozone is a toxic gas. The majority of the harmful effects of hydrocarbon pollution are not due to the hydrocarbons themselves, but the hydrocarbons and other organic compounds (VOCs) present in the atmosphere oxidize through a series of chemical and photochemical reactions, as a result many noxious secondary pollutants like ozone & others (photochemical smog) are produced, which are harmful to animals, plants and human.
Arsenic(As)
Arsenic is a naturally occurring element with atomic number 33. It occurs as an impurity in many ores and in coal. It is also found in the natural environment in some abundance as well as in small quantities in rock, soil, water and air. It is present in many different minerals. It is present in the environment both naturally and due to certain human activities. This is a notoriously poisonous metalloid that has many allotropic forms; yellow, black and grey are a few that are regularly seen. Pure arsenic is a gray metal-like material usually found combined with other elements such as oxygen, chlorine, and sulphur. Arsenic and its compounds are used as pesticides, herbicides, insecticides and various alloys. Arsenic in animals and plants combines with carbon and hydrogen to form organic arsenic compounds. Most inorganic and organic arsenic compounds are white or colorless powders that do not evaporate. They have no smell, and most have no special taste. Most arsenic compounds can also dissolve in water. Arsenic cannot be destroyed in the environment. It can only change its form. Arsenic in air will settle to the ground or is washed out of the air by rain. Many arsenic compounds can dissolve in water.
Industrial processes such as mining, smelting, coal-fired power plants, burning vegetation and volcanoes all contribute to the presence of arsenic in air, water and soil. Environmental contamination also occurs because it is used in agricultural pesticides, preparation of chemicals for timber preservation, paints, dyes, metals, soaps, insecticides and semi-conductors. Apart from its natural occurrence it is also released into the environment through burning fossil fuels, paper production, cement manufacturing also.
Lead(Pb)
Lead molecular formula: Pb, is a naturally occurring metal found in small amounts in the earth’s crust and most widely used metal after iron. It is a extremely toxic and poisoning heavy metal. There are several uses of lead such as in manufacturing of lead acid batteries, X-ray equipment, lead sheet and pipes, power cables, in brass and bronze alloys, glass making, ceramic glazes, pigments and other paint additives, additions to PVC and an additive in gasoline. The battery industry is now the principle consumer of lead using an estimated 76% of annual primary and secondary lead produced. Lead in gasoline has been the major source of lead emissions to the environment but is now being phased out almost universally and therefore the production and recycling of lead acid batteries is now becoming the most significant source of lead exposure in the country.
Lead can enter water, air and soil from natural and anthropogenic sources and can cause adverse effects on human body and lead poisoning is a serious health risk to children. Its sources in ambient air are auto exhaust (from gasoline),paints, storage batteries etc. Bihar is rapidly developing its solar power industry considering solar to be a perfect energy option, but according to a new study, side effects of solar power projects can cause lead pollution to our environment.
Nickel(Ni)
Nickel- molecular formula: Ni, is a silvery-white, hard, malleable, ductile, ferromagnetic heavy metal. It is a good conductor of electricity and heat .Atomic number: 28, atomic mass: 58.7 and melting point: 1453°C. Nickel metal is relatively resistant to corrosion. It dissolves in dilute mineral acids and is insoluble in concentrated nitric acid. The vast majority of nickel is used in alloys. It is used to make stainless steel and other metal alloys. It is commonly alloyed with iron, copper (Monel), chromium, aluminium and zinc. Alloys are used in the making of metal coins, jewellery and, in industry, for making metal items. Nickel and nickel compounds are used for nickel electroplating, to color ceramics, to make batteries, for permanent magnet materials, and as catalysts. Nickel acetate is used mainly as a mordant in the textile industry also.
It is also a major toxic metal. Its sources in ambient air are combustion of coal, diesel and residual oils, tobacco smoke, chemicals and catalyst, steel and non-ferrous alloys manufacture. Cigarette smoke also contains significant quantities of nickel. Nickel was also found in the air as smallest particulate matter emitted from coal-fired power plants, combustion of petroleum coke at refineries and from metal industry.
COMPILATION OF AIR POLLUTANTS, THEIR SOURCES AND EFFECTS
ANNEXURE 1
AIR POLLUTANTS, THEIR SOURCES AND EFFECTS |
Pollutant |
Possible Sources |
Effects |
Natural |
Anthropogenic |
Human/flora/fauna |
Environment & Property |
Sulphur dioxide (SO2)
SO2 is the chemical compound produced by volcanoes and in various industrial precursor and are also a precursor to particulates in the atmosphere. |
Volcanos (67%) |
combustion of fossil fuel (coal, heavy fuel oil in thermal power plants, office, factories)
Paper Industry
extraction & distribution of fossil flues
smelting of metals (sulfide ores to produce copper, lead and zinc)
Petroleum refining
combustion process in diesel, petrol, natural gas driven vehicles |
respiratory illness
visibility impairment
aggravate existing heart and lung diseases |
acid rain
aesthetic damage |
Oxides of Nitrogen (NOx)
Oxides of nitrogen are a generic term for a group of highly reactive gases that contain nitrogen and oxygen in varying amounts. NOx are emitted as nitrogen oxide (NO) which is rapidly dioxide (NO2) Nitrogen dioxide (NO2) is a reddish-brown toxic gas with a characteristic sharp, biting odor and is a prominent air pollutant.
|
Lightning
Forest fires
Bacterial activity of soil |
High temperature combustion (internal combustion engines, fossil fuel-fired power stations, industrial)
Burning of Bio-mass and Fossil Fuels |
irritates the nose and throat
increase susceptibility to respiratory infections |
Precursor of ozone formed in the troposphere
Form atmospheric fine particulate matter burden as a result of oxidation to form nitrate aerosol |
Pollutant |
Possible Sources |
Effects |
Natural |
Anthropogenic |
Human/flora/fauna |
Environment & Property |
Respirable Suspended Particulate Matter
(PM10, size< 10 µm, coarse fraction PM 10 - PM2.5). called thoracic fraction)
Particulate natter (PM) is a complex mixture of suspended solid and liquid particle in semi equilibrium with surrounding gases. The major constituents of RSPM are organic and elemental carbon, metals/element like silicon, magnesium, iron. ions like sulphates, nitrates, ammonium etc. PM10 can settle in the bronchi and lungs and cause health problems |
Coarse particles are produced by the mechanical break-up of larger solid particles.
Wind blown dust as road dust, fly ash, soot, agricultural processes
Physical processes of crushing. grinding and abrasion of surfaces.
photochemical produced particles, such as those found in urban haze
Pollen grains, mould spores, and plant and insect parts
Non-combustible materials released when burning fossil fuels. |
Road traffic emissions particularly from diesel vehicles
Industrial combustion plants some public power generation
Commercial and residential combustion
Non-combustion processes (e.g. quarrying)
agricultural activities |
cardio-pulmonary problems
asthma, bronchitis, and pneumonia in older people |
|
Particulate Matter 2.5 (PM2.5' size < 2.5 m, fine fraction size up to 2.5 µm, respirable fraction Airborne particles smaller than 2.5 µm called fine particles.
Composed mainly of carbonaceous materials (organic and elemental), inorganic compounds (sulfate, nitrate, and ammonium), and trace metal compounds (iron, aluminium, nickel copper zinc, and lead). pose the greatest problems, PM 2.5' tend to penetrate into the gas exchange regions of the lung, and very small particles(< 100 nanometers) may pass through |
Fine particles are largely formed from gases.
Ultrafine particles are formed by nucleation, which is the initial stage in which gas becomes a particle. These particles can grow up to a size of 1 µm either through condensation, when additional gas condensates or coagulation |
Vehicular emission
Industrial combustion plants some public power generation
Commercial and residential combustion |
oxidative stress
respiratory symptoms such as irritation of the airways, coughing, or difficulty breathing
decreased lung function
aggravated asthma
chronic bronchitis
irregular heartbeat cardio-pulmonary disordera
premature death in people with heart or lung disease
|
aesthetic damage
Visibility reduction |
Pollutant |
Possible Sources |
Effects |
Natural |
Anthropogenic |
Human/flora/fauna |
Environment & Property |
The lunges to affect other organs. The smallest particles, however less than 100 nm (nanoparticles) can get into the bloodstream and effect the cardiovascular system |
|
|
|
|
Ozone (O3)
Ozone is a pale blue gas, soluble in water and non-polar solvents with specific sharp odor somewhat resembling chlorine bleach.
Ozone is a secondary pollutants formed in the atmosphere by reaction between oxides of nitrogen and volatile organic compounds (VOCs) in the presence of sunlight. Peak O3 levels occur typically during the warmer times of the year. |
ozone is present in the atmosphere in the stratosphere, in a region also known as the ozone layer between about 10 km and 50 km above the surface |
formed by the reaction of sunlight on air containing hydrocarbons and nitrogen oxides emitted by car engines industrial operations, chemical solvents to form ozone
electronic equipment such as photocopiers |
lung function deficits
respiratory illness
Premature death, asthma, bronchitis, heart attack, and other cardiopulmonary problems.
ground-level ozone and pollution which interferes with photosynthesis and stunts overall growth of some plant species |
Ozone cracking in car tires, gaskets, O-rings is caused by attack of ozone on any polymer possessing olefin or double within its chain structure,
ozone present in the upper troposphere acts as a greenhouse gas, absorbing some of the infrared energy emitted by the earth. |
Lead
Lead is a bright silvery soft dense, ductile, highly malleable, bluish-white metal that has poor electrical conductivity heavy metal and is highly resistant to corrosion. |
food (lead is absorbed by plants) |
Waste incineration
Metal processing
Paint Industry
lead solder in food cans, breast milk, drinking water Cosmetics, Ceramic pottery, brining of firewood or kerosene, indigenous remedies, tobacco and tobacco products, contaminated drinking water, toys, industrial effluents, lead acid batteries, ammunition, paints and |
Pb is rapidly absorbed into the bloodstream and is believed to have adverse effects on the central nervous system, the cardio vascular system, kidneys, and the immune system |
|
Pollutant |
Possible Sources |
Effects |
Natural |
Anthropogenic |
Human/flora/fauna |
Environment & Property |
|
|
Automobile exhaust. |
- Accumulates both in soft tissues and the bones.
Causes nephropathy, and colic-like abdominal pains.
Weakness in fingers, wrists, or ankles.
Miscarriage and reduction of fertility in girls
permanently reduce the cognitive capacity of children |
|
Carbon monoxide (CO)
also Calles carbon us oxide , is a colorless, odorless and tasteless gas which is slightly lighter than air, It is slightly toxic to humans and animals in higher quantities. Mainly formed by incomplete combustion of carbon containing fules.
|
produced during normal animal metabolism (by the action of home oxygenize 1 and 2 on the home from hemoglobin break down and produces carboxyl hemoglobin in normal persons)in low quantities and has some normal biological function (signaling molecule)
volcanic activity
forest and bushfires |
Exhaust of internal combustion engines, especially of vehicles with petrol engines
Burning of carbon fuels
organic combustion in waste incineration
power station processes
Iron smelting
burning of crop residues |
Co enters the blood stream through lungs and combines with hemoglobin forms carboxyhemoglobin. This condition is known as anorexia, which inhibits blood's oxygen carrying capacity to organs and tissues.
Person's with heart disease are sensitive to CO poisoning and may experience chest pain if they breathe the gas while exercising.
adverse effects on the fetus of a pregnant woman |
|
Pollutant |
Possible Sources |
Effects |
Natural |
Anthropogenic |
Human/flora/fauna |
Environment & Property |
|
|
|
Infants, elderly per sibs, and individuals with respiratory diseases are also particularly sensitive.
anti-inflammatory, vasodilators and encouragers of neovascular growth |
|
Ammonia (NH3)
A compound of nitrogen and hydrogen, a colorless gas with a characteristic pungent odour. Contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers and either directly or indirectly, is also a building block for the synthesis of may pharmaceuticals. |
putrefaction of nitrogenous animal and vegetable matter
Ammonia and ammonium salts are also found in small quantities in rainwater, fertile soil and in seawater
during volcanic eruption
The kidneys secrete NH3 to neutralize excess acid |
Farms
Fertilizers Industry
Industrial sites that store ammonia or use it as a refrigerant can release high levels if the chemical leaks or is pilled |
irritating to skin, eyes, throat, and lungs and cause coughing
burns
Lung damage and death may occur after exposure to very high concern tractions of ammonia |
|
Benzene (C6H6)
Benzene is a colorless, sweet smelling liquid. Benzene is generated whenever carbon-rich materials undergo incomplete combustion. Benzenes is federated whenever carbon-rich materials undergo incomplete combustion. |
volcanoes
forest fires |
Combustion of fuel (automotive fuel, wood and stationary fossil fuel, other aromatics
evaporation (fuel storage containers, during refueling
Industrial emission
Coke oven
Perchlorethlyene is emitted from some dry cleaning facilities |
Hematologic, neurotoxin, leukemogenic, carcinogenic effects
Chronic exposure to benzene may cause chromosomal damage, immune suppression, a plastic anemia, myelodysplastic syndrome, leukemia,
|
|
Pollutant |
Possible Sources |
Effects |
Natural |
Anthropogenic |
Human/flora/fauna |
Environment & Property |
|
|
tobacco smoke, wood smoke
glues, paints, furniture wax. and detergents |
- non-Hodgkin's lymphoma, and cancer of the lung and nasopharynx
Effect the Reproductive system, developing fetus and fertility in men, low birth weights, delayed bone formation, and bone marrow damage |
|
Polyaromatic hydrocarbons (BaP) (particulate phase only)
is a five-ring polycyclic aromatic hydrocarbon whose metabolites are mutagenic and highly carcinogenic |
coal tar (after a forest fire).
eruption of volcanoes |
Incomplete combustion of fuels (Processing of coal and crude oil)
Combustion of natural gas
Road transport
Industrial plant
Tobacco smoke
coal tar
automobile exhaust fumes (especially diesel engines), in all smoke resulting from the combustion of organic material
charbroiled food, burnt toast, cooked meat products, in burnt foods such as coffee |
Mutagenic and highly carcinogenic (skin, lung, and bladder cancer in humans and in animals)
skin rash or eye irritation
Bronchitis |
|
Pollutant |
Possible Sources |
Effects |
Natural |
Anthropogenic |
Human/flora/fauna |
Environment & Property |
Arsenic (As)
is a solid layered, a ruffled analogue of graphite, metallic fray in color and is a semiconductor. It is a potent poison |
volcanic ash, weathering of the arsenic containing mineral and ores as well as groundwater.
food, water, soil and air |
Smelting of metals,
Combustion of fuels(especially of low grade brown coal)
Use of pesticides. |
epigenetic changes
multi-system organ failure
As poisoning |
|
IARC)
recognizes arsenic and group 1 carcinogen (IARC) |
|
wood preservation, glass production, nonferrous metal alloys, electronic semiconductor manufacturing.
coke over emissions assoc- coated with the smelter industry |
|
|
Nickil (Ni)
a silvery-white lustrous corrosion-resistant metal with a skylight golden tinge |
urease (an enzyme which assists in the hydrolysis of urea) contains nickel |
Combustion of fossil furls
Nickel plating
Metallurgical processes |
Nickel sulfide fume and dust is believed to be carcinogenic
allergy, dermatitis, Sensitivity to nickel may also be present in patients with pompholyx. |
explosive in air |
Air Quality Monitoring Network
All the air quality monitoring programmes in the State are carried out by the State Pollution Control Board. There are 9 Divisions and 38 districts in the state. The cities/towns like Patna, Muzaffarpur, Chapra, Motihari, Siwan, Gopalganj, Samastipur, Madhubani, Munger, Bhagalpur, Purnea, Darbhanga, Betiah and Sasaram are the district head quarters and have significant population more than one lac. Rajgeer in district- Nalanda and Bodhgaya in district-Gaya are tourist places of international level and have their historic importance. Vehicular emissions, industrial emissions and other development & human activities around these cities/towns play significant role in the quality of ambient air.
At present, there are only two air quality monitoring stations are operational in the state, one at Beltron Bhawan, Patna and another at Gandhi Maidan, Patna. In addition, three monitoring stations namely at: Bodhgaya(Gaya), Barauni(Begusarai) and Muzaffarpur under NAMP are likely to be put into operation very shortly. The State Pollution Control Board has also planed to establish xyz additional new air monitoring stations with the support of state govt. at: Bhagalpur, Purnea, Darbhanga, Rajgeer, Betiah and Sasaram. The scheme is under progress.
Continuous Ambient Air Quality Monitoring Station(CAAQMS)
The State Pollution Control board has established continuous air quality monitoring station at Indira Gandhi Science Complex-Planetarium, Patna, It has been inaugurated by the Honbl’e Dy Chief Minister Sri Shushil Modi in July 2011 and is operational now. The State Board is planning to establish more such monitoring stations in every districts of the state.
|